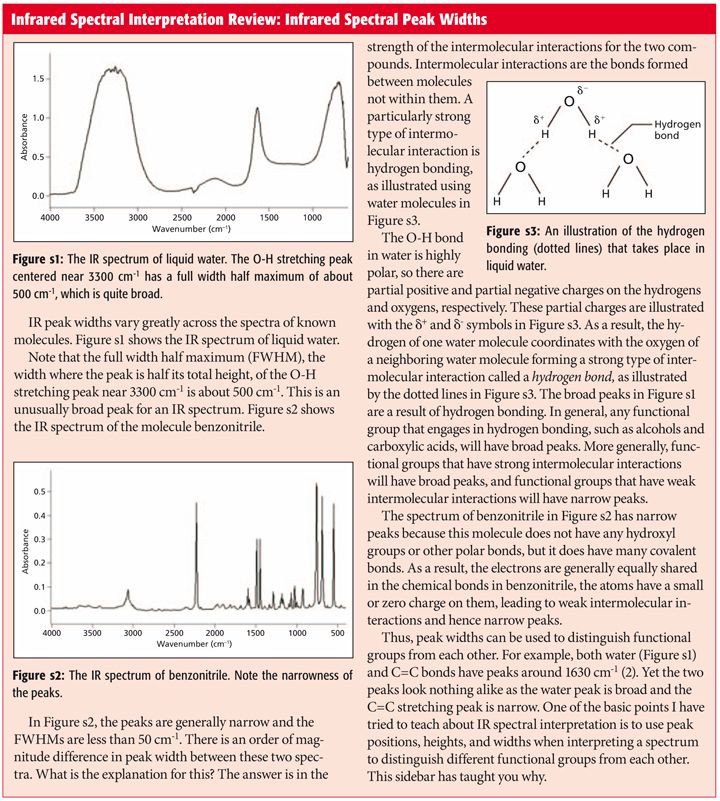
carboxylate ir stretch
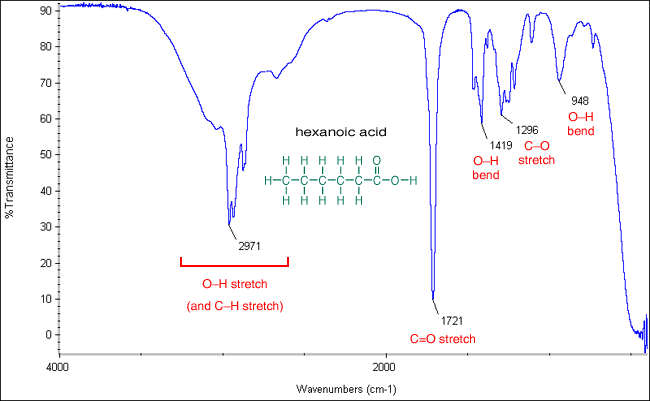
Weatherability of pastel and dark colored rigid PVC compound can be achieved by substituting the more light. 1780 - 1710 s 1690 - 1630 s The carbonyl stretching absorption is one of the strongest IR absorptions and is very useful in structure determination as one can determine both the number of carbonyl groups assuming peaks do not overlap but also an estimation of which types.
Carboxylate anions exhibit characteristic vibrational spectra in the infrared IR which typically includes an intense peak due to symmetric stretching of the COO functional group as well as a peak due to antisymmetric stretching.

. This attribute is why the pair of carboxylate stretching peaks are so intense. Spectra were recorded in regions below 800 cm 1 8001500 cm 1 the fingerprint region the region between 2800 and 3000 cm 1 C-H stretch region and finally the region between 3000 and 3600 cm 1 O-H stretch region by method as mentioned by Belton et al. Abstract A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal center can be used to infer if the geometry of the bonding is monodentate or bidentate.
The frequency of the carboxylate stretching vibration near. The analysis is performed by assigning the value of the CO stretching wavenumber to a particular range characteristic of each type of compound. We have tested this principle with ab initio modeling for aqueous metal carboxylate complexes and have shown that it does indeed hold.
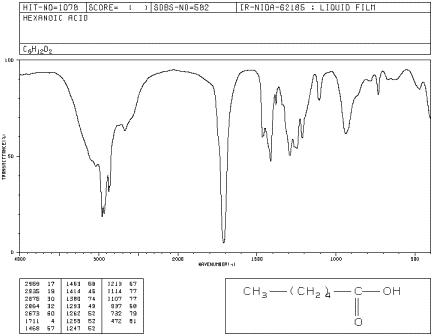
So lets look at an IR spectrum just a generic IR spectrum for an acid and hydride. 1 in the dianion of 4-hydroxybenzoic acid to 1650 cm. Finally lets look at one more example of a symmetric and asymmetric stretch and thats an acid and hydride.
We report the first IR spectroscopic observation of carboxylate stretching modes in free space ie in the complete absence of solvent or counterions. So we know this is our carbon-hydrogen in here. A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal center can be used to infer if the geometry of the bonding is monodentate or bidentate.
Thus ketones are said to inhabit the range of 1715-1740 cm -1 and simple esters come at 1740-1760 cm -1 some 20-30 cm. When the bonds of the -CO 2 group stretch asymmetrically and symmetrically there is a large value of dµdx. Gas-phase spectra of a series of benzoate anions have been recorded and compared to condensed-phase spectra revealing the profound influence of the environment on the symmetric and antisymmetric carboxylate stretch modes.
Gas-phase spectra of a series of benzoate anions have been recorded and compared to condensed-phase spectra revealing the profound influence of the environment on the symmetric and antisymmetric carboxylate stretch modes. This area is sometimes referred to as the carbonyl stretching region as a result. Lets draw a line around 1500.
We report the first IR spectroscopic observation of carboxylate stretching modes in free space ie in the complete absence of solvent or counterions. To divide our two regions we draw a line around 3000 here. The infrared spectra of six aqueous carboxylate anions have been calculated at the m05-2xcc-pvtz level of theory with the smd solvent model and validated against experimental data from the literature over the region of 1700 cm -1 to 1250 cm -1.
IR carboxylate stretching modes are widely used to infer if the geometry of the bonding is monodentate or bidentate. In theory one could identify the metal ion. University of Central Missouri.
We have tested this principle with ab initio modeling for aqueous metal carboxylate complexes and have shown that it does indeed hold. In fact different types of attachments like ionic and coordination bonds monodentate bidentate bridging and bidentate chelating can be interpreted by observing antisymmetric stretching of. This region corresponds to the stretching modes of the carboxylate group and is often.
3700 - 3500 m. Carbonyl stretching peaks generally fall between 1900 and 1600 cm -1 assume all peak positions hereafter are in wavenumber units a relatively unique part of the IR spectrum.
The C O Bond Part Iii Carboxylic Acids
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts

Lec15 Ir Spectra Of Carboxylic Acids Amides And Esters Youtube
21 3 Spectroscopy Of Carboxylic Acids Chemistry Libretexts

Interpretation Of Carboxylic Acid Ir Spectra Youtube
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts

How Range Peak Ir Spectrum For Carboxylic Acid Salts Ex Calcium Lactate
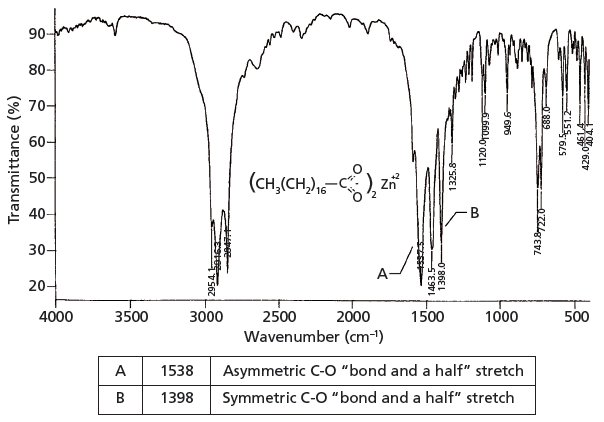
The Carbonyl Group Part V Carboxylates Coming Clean

How Range Peak Ir Spectrum For Carboxylic Acid Salts Ex Calcium Lactate

Infrared Spectrum Of Ethanoic Acid Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Ethanoic Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes

Infrared Spectrum Of Benzoic Acid C7h6o2 C6h5cooh Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Benzoic Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts
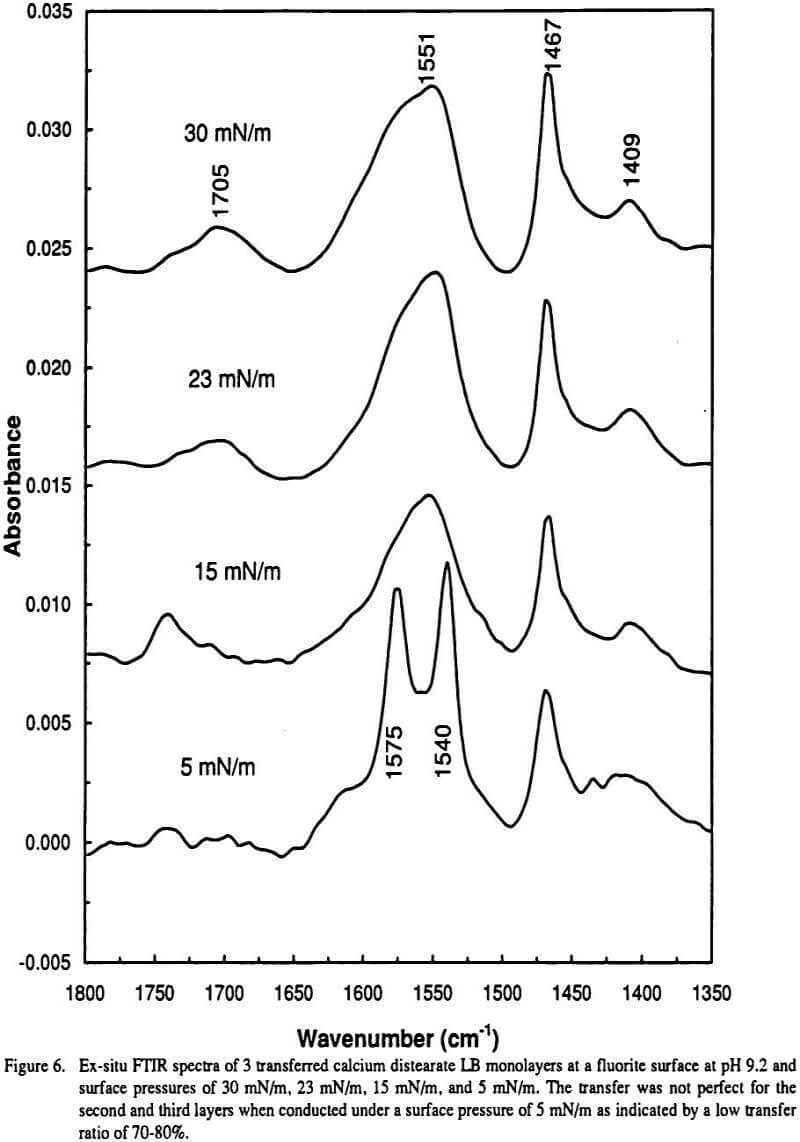
Ir Infrared Absorption Bands Of Carboxylate
The C O Bond Part Iii Carboxylic Acids
The C O Bond Part Iii Carboxylic Acids

0 Response to "carboxylate ir stretch"
Post a Comment